Claim trial deaths were linked to RSV drug is misleading
Kate Atkinson
November 18, 2024
WHAT WAS CLAIMED
Twelve babies died during clinical trials of a new medicine to protect against RSV.
OUR VERDICT
Misleading. The deaths were not related to the RSV medicine.

AAP FACTCHECK – The deaths of 12 infants during a clinical trial for a respiratory syncytial virus (RSV) drug were not caused by the shot, despite misleading claims online.
It’s true that a dozen babies died during the clinical trial period, but none of the deaths were caused by the drug, which is administered via injection.
In the third and fourth trials, the rate of death between babies who received the dose and a control group which was given a placebo was the same.
The claim appears in Facebook posts sharing the headline of an article published by the Children’s Health Defence, an organisation that advocates against vaccines.
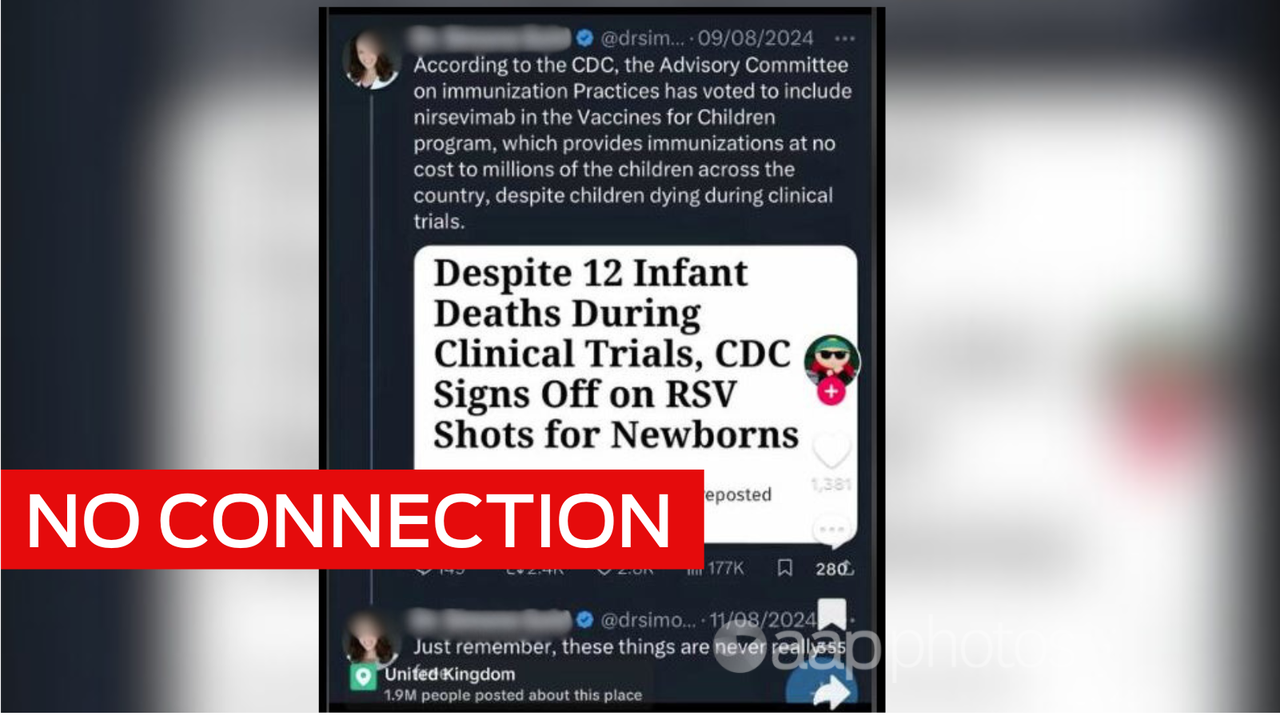
“Despite 12 Infant Deaths During Clinical Trials, CDC Signs Off on New RSV Shot for Newborns,” it reads.
The August 4 2023 article was published after the US Food and Drug Administration (FDA) approved Beyfortus – also known as nirsevimab – a monoclonal antibody drug used to prevent newborns catching RSV.
The product has also been approved by Australia’s Therapeutic Goods Administration.
In November 2024 the Australian federal government announced that a maternal RSV vaccine called Abrysvo would be available for free from next year.
States are also widening access to Beyfortus for newborns.
RSV is a virus that causes respiratory infections and mostly affects newborns or elderly people. It’s a leading cause of childhood hospitalisation.
A monoclonal antibody differs from a vaccine because it directly delivers antibodies against a virus, instead of teaching the body how to fight off future infections.
Its approval followed extensive clinical trials that involved thousands of babies across multiple countries.
Across three clinical trials, deaths were reported for 11 babies who received Beyfortus, one baby who had received an existing RSV drug called palivizumab, and three who had received a placebo.
Table 27 of an FDA briefing document provides details about the underlying comorbidities and causes of death (page 95).
The causes of death for 11 infants who received Beyfortus included pulmonary vein stenosis, diarrhoea, acute gastroenteritis, injuries sustained in a car accident, COVID-19, acute bronchiolitis, bronchopneumonia, cardiac shock and congestive heart failure.
The cause of death for two babies was listed unknown, but one was born very premature and the other had a suspected undiagnosed chronic illness.
The child who received palivizumab died from bronchiolitis, while pneumonia and pericardial effusion was listed as the cause of death for the babies who had received the placebo.
The document confirmed that none of the deaths were related to receiving the Beyfortus dose.
“There were no trends associated with reported events and the cause of death can be attributed to underlying medical condition or common cause of infant mortality reported in the region where the infant was enrolled,” the document states (page 93).
The document notes that in the third and fourth trials, which included both drug and placebo groups, the rate of death was the same at 0.2 per cent (page 20).
“Deaths occurred with the same incidence (0.2 per cent) in the nirsevimab (6 subjects) and placebo group (3 subjects) and none were considered related to [nirsevimab],” it said.

Annette Regan, an associate professor of epidemiology at the University of San Francisco, said the safety data was strong and clearly showed no effect of Beyfortus on infant death.
“The mortality rate was less than one per cent in most trials which is either similar or lower than the expected infant mortality rate for countries participating in the trials,” she said.
“Infant mortality was also balanced across groups (meaning similar rates in those that got nirsevimab and those that got placebo),” she said.
Allen Cheng, a professor of infectious disease at Monash University, agreed.
“On closer examination, none of the deaths were felt to be related to nirsevimab (Beyfortus) or RSV,” he said.
Assoc Prof Regan also noted that Beyfortus provides pre-made antibodies and does not activate the infant’s immune system. (AAP)



